| Background | Diffraction | Crystal Quality | Facilities |
| Home |
Proteins |
| Neural Networks |
Where is Protein Crystallography Done?
Protein Crystallography research is conducted at Synchrotron Radiation facilities such as the National Synchrotron Light Source at Brookhaven National Laboratory. Synchrotron Radiation is an intense variety of electromagnetic radiation created by accelerating electrons. In order to have the capability of resolving individual atoms, X-rays must be used because of their short wavelength. X-ray beams are created by the Synchrotron and then focused onto a protein crystal mounted in a diffractometer.
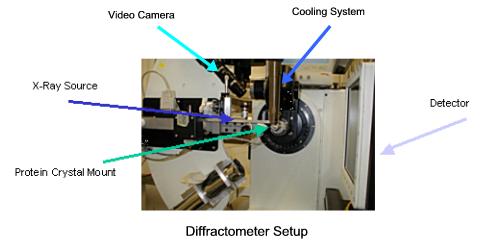
X-rays from the source, the Synchrotron beam line, pass through the crystal and are then collected by the CCD detector. CDD (charged couple device) detectors convert photons to digital images; they are identical to the units found in digital cameras. In order collect sufficient data to determine the structure of the protein, the crystal must be rotated. The crystal mount is automated and can rotate to within a fraction of a degree. A closed circuit video camera monitors the status of the crystal and helps align the crystal in the X-ray beam. Synchrotron X-rays are extremely intense. When they strike protein molecules, they disrupt the molecular bonds and cook the crystal. To prolong the life of the crystal, a jet of liquid nitrogen from the cooling system keeps it at comfortable 100 degrees Kelvin.